Providing Hope to Patients with Carcinomatosis: Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy
For patients with peritoneal dissemination of an abdominal malignancy (e.g., carcinomatosis), cytoreductive surgery (CRS) paired with hyperthermic intraperitoneal chemotherapy (HIPEC) can significantly increase survival time.1 According to Colette Pameijer, MD, FACS, associate professor of surgery, “The CRS/HIPEC technique offers hope to these patients. It adds a significant amount of time to their lives, which would otherwise be counted in months, rather than years.” Metastatic cancer cells implanted within the peritoneal cavity or its surfaces are not detectable by CAT scan, and do not respond well to standard resection and systemic chemotherapy. Patients with carcinomatosis who receive palliative treatment have a median survival time of six months.1
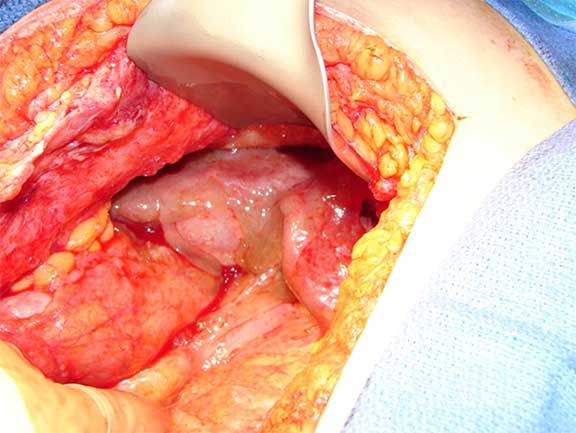
Mucin covering the liver
Dr. Pameijer has performed approximately 45 CRS/HIPEC procedures while at Penn State Health Milton S. Hershey Medical Center. “With CRS/HIPEC, a layer of the peritoneal lining containing cancerous cells is removed. We then perfuse the region for about 90 minutes with a chemotherapy solution heated to 40-41° C,” she explains. Dr. Pameijer typically uses mitomycin Celsius, although her choice of chemotherapy is guided by tumor type; other options include oxaliplatin. “The procedure is rigorous (3-12 hours total). Ideally, referral should occur early, when a patient with a primary abdominal cancer [e.g., ovarian, colorectal, gastric, appendiceal] shows increased serum tumor marker and has a small volume of peritoneal cancer not yet detectable on X-ray or CAT scan,” remarks Dr. Pameijer. With CRS/HIPEC, the drug is in direct contact with tumor cells at concentrations much higher than can be safely achieved with systemic administration; the heat alone is cytotoxic and potentiates the action of many chemotherapy drugs.
After CRS/HIPEC, median survival is approximately 29 months and survival curves resemble those seen in patients with resected liver metastases.1 Dr. Pameijer notes, “CRS/HIPEC is really only one component of what should be a comprehensive treatment regimen, including standard chemotherapy.” Wider use of CRS/HIPEC is likely to emerge in the future, given current organized efforts to develop standardized protocols that define chemotherapy perfusion parameters.2

Colette R. Pameijer, MD
Associate Professor of Surgery
Phone: 717-531-5272
Email: cpameijer@pennstatehealth.psu.edu
Fellowship: Surgical oncology, City of Hope National Medical Center, Duarte, Calif.
Residency: General surgery, University of Wisconsin Hospitals and Clinics, Madison, Wis.; General surgery, Hahnemann University Hospital, Philadelphia, Pa.
Medical School: Medical College of Pennsylvania/Drexel University - School of Medicine, Philadelphia, Pa.
Connect with Colette R. Pameijer, MD, on Doximity
References:
- Levine EA, Stewart JH 4th, Shen P, Russell GB, Loggie BL, Votanopoulos KI. 2014. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. Apr;218(4):573-85.
- Turaga K,Levine E, Barone R, et al. 2014. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. May;21(5):1501-5.

