Prophylactic Total Gastrectomy to Manage Hereditary Gastric Cancer Risk
Minimally invasive surgical treatment options are helping to revolutionize surgical care for patients with many upper gastrointestinal cancers and pre-cancerous syndromes at Penn State Health. Hereditary Diffuse Gastric Cancer (HDGC) is an inherited, autosomal dominant syndrome with high (80%) penetrance which results in invasive stomach cancers (often multifocal) at a relatively young age (30-50 years old)1. Mutations in the E-cadherin (CDH1) are usually the cause of HDGC and patients often have a strong family history of stomach cancer and breast cancer (lobular breast cancer is also associated with CDH1 mutations)2.
“Prophylactic total gastrectomy early in adulthood is recommended for patients with a CDH1 mutation to prevent the development of gastric cancer, which can occur in 70-80% of patients with this genetic mutation,” says Niraj J. Gusani, MD, MS, FACS, Director for the Program for Liver, Pancreas, & Foregut Tumors at the Penn State Cancer Institute. Women often postpone prophylactic gastrectomy until after full development or childbearing, while men often undergo the procedure soon after a positive gene test. For women, mastectomy is often recommended as well, but usually is less urgent than gastrectomy.
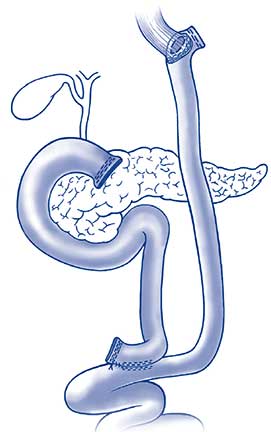
Roux-Y reconstruction with esophagojejunostomy. ©Steinemann et. al.; licensee BioMed Central Ltd. 2011.
At Penn State Cancer Institute, total gastrectomies for such patients are routinely performed using a minimally-invasive robotic-assisted approach, which reduces recovery time and the risk for post-operative infection. Dr. Gusani, who also serves as Associate Professor of Surgery, Medicine, and Public Health Sciences, notes, “Gastrectomy is the recommended approach, because surveillance is unreliable. The gastric lining is difficult to accurately and completely assess, and even surveillance endoscopy frequently fails to detect precancerous or cancerous lesions.” Postoperative recovery with total gastrectomy includes temporary placement of a feeding tube, which is removed once adequate hydration and ability to maintain body weight are achieved with oral feeding. Most patients go on to lead very normal lives including eating all types of foods3. Some changes to the patient’s eating routine, however, may persist for the rest of his or her life. “Because reconstruction after total gastrectomy involves creation of only a small intestinal pouch as a reservoir for swallowed food (Figure), patients can eat, but must limit the quantity of food they can ingest at any one time. Bile reflux also occurs in many patients and must be managed for patient comfort. Patients shouldn’t lie flat and beds can be adjusted so that the upper body is elevated,” notes Dr. Gusani. Patients undergoing total gastrectomy may also require routine lab monitoring of iron levels and will need to receive monthly vitamin B12 shots.
Patients with mutations in genes other than CDH1 can be considered appropriate candidates for prophylactic gastrectomy, although this is quite rare. According to Dr. Gusani, “The link between other genes and gastric cancer is still unclear and an aggressive procedure like total gastrectomy for risk reduction in such instances is controversial. For these patients, surveillance with annual upper endoscopy and biopsy of suspicious tissue may be a more reasonable approach.”
Although minimally invasive stomach resection can be attempted in any patient needing gastric surgery, it may not be possible in all situations. In many patients with gastric cancer (most cases of gastric cancer occur in patients without genetic mutations or predisposition), the tumors are often detected in the advanced stage. These patients may require gastrectomy, but sometimes cannot undergo a minimally invasive procedure. Dr. Gusani concludes, “With advanced gastric cancer, the tumor may be bulky, invading another organ or causing blockage. In these cases, a traditional open procedure is necessary.”

Niraj J. Gusani, MD
Associate Professor of Surgery, Medicine and Public Health Sciences
Penn State College of Medicine and Penn State Cancer Institute
Phone: 717-531-6261
Email: ngusani@pennstatehealth.psu.edu
Fellowship: Surgical Oncology Research, Memorial Sloan-Kettering Cancer Center, New York, NY; Surgical Oncology, University of Pittsburgh Medical Center, Pittsburgh, Pa.
Residency: Surgery, General, University of Chicago Medical Center, Chicago, Ill.
Medical School: University of Pennsylvania School of Medicine, Philadelphia, Pa.
Connect with Penn State Health Gastroenterology and Hepatology on Doximity
References
- Tan RY, Ngeow J. “Hereditary diffuse gastric cancer: What the clinician should know.” World J Gastrointest Oncol. 2015 Sep 15;7(9):153-60. doi: 10.4251/wjgo.v7.i9.153.
- van der Post RS, Vogelaar IP, et al. “Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers.” J Med Genet. 2015 Jun;52(6):361-74. doi: 10.1136/jmedgenet-2015-103094. Epub 2015 May 15.
- Haverkamp L, van der Sluis PC, et al. “Prophylactic Laparoscopic Total Gastrectomy with Jejunal Pouch Reconstruction in Patients Carrying a CDH1 Germline Mutation.” J Gastrointest Surg. 2015 Dec;19(12):2120-5. doi: 10.1007/s11605-015-2963-4. Epub 2015 Oct 6.

