Minimally Invasive and Maximally Effective Approaches to Pancreatic Fluid Collections and Pancreatic Necrosis
Severe necrotizing pancreatitis represents an enormous clinical problem due to its prevalence, economic costs and high levels of morbidity and mortality. After an initial period characterized by systemic inflammatory response, the leading cause of mortality stems from infection of the pancreatic necrosis which develops in 40-70 percent of patients. 1 Without intervention, the mortality rate of this group is exceedingly high, but can be significantly reduced using various methods of surgical pancreatic necrosectomy. However, with perioperative mortality rates of 10-40 percent and complication rates in excess of 70 percent, conventional pancreatic necrosectomy presents its own problems and is typically approached with trepidation. Additionally, repeated trips to the operating room are often required. 2,3
In recent years, an emerging body of evidence has demonstrated that minimally invasive techniques represent a more attractive approach to pancreatic necrosectomy as they can offer a smaller insult to an already critically ill patient, similar technical outcomes, and lower complication rates. These approaches include transperitoneal or transgastric laparoscopic techniques, endoscopic ultrasound (EUS) guided cystgastrostomy, and interventional radiology methods. Matthew T. Moyer, MD, MS, assistant professor of medicine, Penn State Gastroenterology, explains “Here we approach each case differently, but most commonly rely on EUS-guided transgastric or transduodenal cystgastrostomy to treat these patients.” After initial drainage and lavage, direct debridement of any solid debris can be performed either immediately or at a follow-up procedure depending on the configuration of the abscess and the stability of the patient. This results in a relatively clean cyst that heals in time and, with the addition of ERCP, the goal is to re-establish the exocrine flow through the native duct.” In a published case series of patients who underwent EUS-guided transgastric or transduodenal cystgastrostomy at Penn State Health Milton S. Hershey Medical Center, an 83 percent clinical resolution over four months without complication was achieved. 3
In cases with complex or extensive lesions, however, additional drainage with an interventional radiology or surgical approach may be required. Recently, Moyer and Mathew described a unique percutaneous endoscopic approach to this situation where a flexible endoscope is advanced through a targeted access point, previously made under CT guidance, and is used to debride the infected tissue under direct visualization; a large caliber irrigation tube is then left in place for continual post-op lavage. With this method, the peritoneal cavity is not contaminated and both debridement and lavage are achieved using a single access point. Moyer notes “In cases of severe necrotizing pancreatitis requiring extensive debridement, using a flexible endoscope rather than a rigid laparoscope offers the ability to reach sites of the infected bed not directly in line with the access point and to complete the procedure outside the traditional OR.” By post-op day one, the patient had improved dramatically and all drains were successfully removed at two months in the outpatient setting, and patient was fully recovered. Moyer states that “Although the results of this case are encouraging, a larger series is required to more clearly evaluate the clinical efficacy and safety of this approach.”
One of the challenges in effectively treating patients with necrotizing pancreatitis lies in selecting the correct method for an individual patient. Variations in the target lesion require an individually tailored and flexible approach. Admittedly, some patients still require delayed conventional surgical intervention if their situation fails to resolve. Overall, therapeutic techniques must continue to develop for these patients in need of improved treatment options.
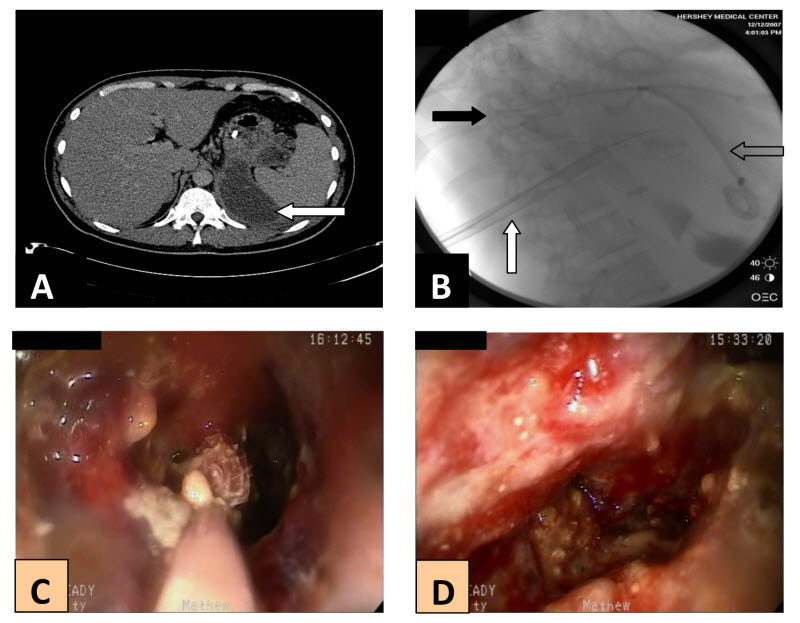
Figure 1. A, initial CT revealing a multi-locular pseudocyst extending from the lesser sac to the splenic hilum (arrow). B, Fluoroscopic image showing the drainage catheter previously placed under CT guidance (black arrow), fluoroscopically placed 30Fr introducer sheath (white arrow), and the previously placed transgastric pig-tailed stents (clear arrow). C, endoscopic view of the Roth net removing necrosum from deep inside the abscess. D, interior of the abscess cavity after removal of a majority of the debris.
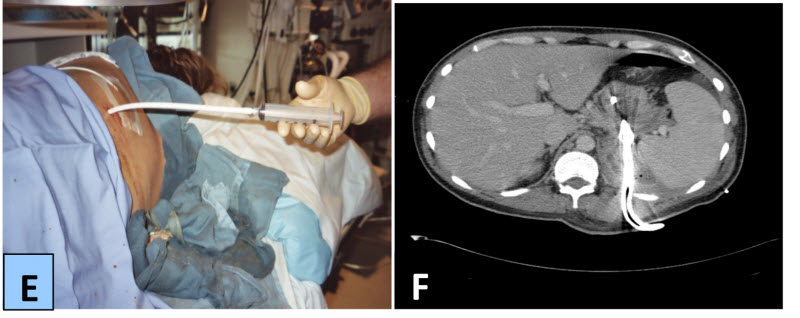
Figure 1. E, placement of the Malecot drain. F, follow-up CT scan demonstrating marked radiographic improvement at 1 month.

Matthew T. Moyer, MD, MS
Professor, Department of Medicine, Division of Gastroenterology and Hepatology, Penn State Cancer Institute
Phone: 717-531-4950
Email: mmoyer@pennstatehealth.psu.edu
Fellowship: Gastroenterology, Penn State Health Milton S. Hershey Medical Center, Hershey, Pa.
Residency: Internal Medicine, Penn State Health Milton S. Hershey Medical Center, Hershey, Pa.
Medical School: Penn State College of Medicine, Hershey, Pa.
Connect with Matthew T. Moyer, MD, MS, on Doximity
References
- Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006; 101:2379.
- Conner S, Alexakis N, Raraty GT, et al. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499-505.
- H Seifert, M Biermer, W Schmitt, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009 58: 1260-1266.

