WATCHMAN™ Device: Innovative Therapy for Stroke Prevention in Growing Atrial Fibrillation (AF) Population
By the year 2050, it is estimated that nearly 16 million people could suffer from atrial fibrillation (AF), but not all will tolerate long-term anticoagulant therapy.1 The WATCHMAN™ Left Atrial Appendage Closure (LAAC) device, the latest minimally invasive option at the Heart and Vascular Institute, can help bridge this clinical gap. In March, Penn State Health Milton S. Hershey Medical Center became the first in the region to implant the WATCHMAN device in non-valvular AF patients at increased risk for stroke and systemic embolism seeking an alternative to long-term warfarin therapy.
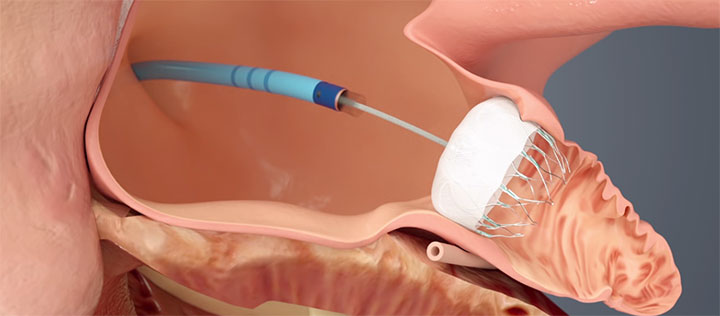
WATCHMAN™ is delivered via a transfemoral approach and is designed to close the left atrial appendage (LAA) to prevent migration of blood clots, thus reducing the risk of stroke and systemic embolism. Images provided courtesy of Boston Scientific.© 2016 Boston Scientific Corporation or its affiliates. All rights reserved.
The PROTECT AF, CAP and PREVAIL trials found the WATCHMAN device comparable to warfarin therapy and superior at preventing stroke and death in eligible patients. The device has demonstrated a 32 percent reduction in all-cause stroke, and greater reductions in rates of hemorrhagic stroke (85 percent), disabling stroke (63 percent) and cardiac death (56 percent).2,3 Rates of warfarin cessation at 12 months among patients in the three clinical trials were more than 93 percent.3 Seven-day procedure and safety-related event rates ranged from 3.8 to 4.8 percent.4
“This groundbreaking device has been shown to be very effective at occluding the left atrial appendage, where most cardiac emboli originate,” says Sarah Hussain, MD, assistant professor, Heart and Vascular Institute, who, along with Soraya Samii, MD, PhD, associate professor, and Mark Kozak, MD, associate professor, perform the procedure.
There are limited alternatives to the WATCHMAN. Other devices, such as the Lariat suture device, have less extensive data available on outcomes and efficacy. “It is important to note that patients who undergo the WATCHMAN procedure will require short-term anticoagulation following implant,” says Dr. Samii.
The WATCHMAN procedure is usually performed in fewer than two hours. Once anesthesia is administered, a transesophageal echocardiogram (TEE) measures the left atrial appendage (LAA) to confirm the appropriate device size. Following trans-septal access, the device is deployed, and TEE and angiography confirm adequate positioning, prior to release of the device. The device permanently seals off the LAA, where most of the clots that cause stroke in AF patients originate. “Post-procedure recovery is generally short: patients will usually only spend one night in the hospital,” says Dr. Hussain.
Data suggests that almost 40 percent of patients with AF and at high-risk for stroke are not anticoagulated, due to concern for bleeding complications. The WATCHMAN device may be a good alternative for a majority of these patients.
With a growing structural heart program at Hershey Medical Center, WATCHMAN is another example of its multidisciplinary approach to heart disease. “Patient management involves specialists from electrophysiology, interventional cardiology and cardiac imaging, as well as a dedicated cardiac anesthesiology team,” Dr. Samii emphasizes.

Soraya M. Samii, MD
Associate Professor of Medicine
Electrophysiologist
Phone: 717-531-3907
Email: ssamii@pennstatehealth.psu.edu
Fellowship: Cardiology, Penn State Health Milton S. Hershey Medical Center, Hershey, Pa.; Cardiology, University of Colorado Health Sciences Center, Denver, Colo.
Residency: Internal medicine, University of Pittsburgh Medical Center Presbyterian, Pittsburgh, Pa.
Medical School: Penn State College of Medicine, Hershey, Pa.
Connect with Soraya M. Samii, MD, on Doximity

Sarah K. Hussain, MD
Assistant Professor, Medicine
Electrophysiologist
Phone: 717-531-3907
Email: shussain1@pennstatehealth.psu.edu
Fellowship: Electrophysiology, University of Virginia School of Medicine, Charlottesville, Va.; Cardiology, University of Virginia School of Medicine, Charlottesville, Va.
Residency: Internal Medicine, Cleveland Clinic Foundation, Cleveland, Ohio
Medical School: Medicine, Aga Khan Medical College, Karachi, Pakistan
Connect with Sarah K. Hussain, MD, on Doximity
References:
- Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011 Nov 1;124(18):1982-93.
- Watchman Device Overview Presentation. http://www.bostonscientific.com/en-US/products/laac-system/watchman-device/download-center.html. Accessed February 16, 2016.
- Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, Huber K, Whisenant B, Kar S, Swarup V, Gordon N, Holmes D; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014; 312(19):1988-98.
- http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/
MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM417177.pdf . Accessed February 15, 2016.
