NanoSENSE: Using wearable diagnostics to manage heart failure
A study aimed at heart failure management using nanotechnology is currently underway at Penn State Health Milton S. Hershey Medical Center and Hackensack Meridian Health, with plans to expand to three more medical centers in the future. The NanoSENSE validation study launched in the summer of 2019 and is a multi-center, prospective, nonrandomized, observational, nonsignificant risk study. The NanoSENSE study will enroll up to 500 subjects in order to collect clinical endpoints which include at least 150 heart failure hospitalizations in participating subjects.
Conducted by Nanowear, a nanotechnology-based connected-care and remote monitoring platform, the study utilizes proprietary and the first-and-only FDA 510(k) cleared cloth-based nanosensors. SimpleSENSE is a monitoring undergarment and closed-loop machine learning platform, which captures and algorithmically scores:
- Physical activity
- Impedance cardiography measuring stroke volume and cardiac output
- Phonocardiography
- Multi-channel electrocardiography assessing heart rate and heart rate variability
- Respiratory rate
- Thoracic impedance
Nanowear received its third FDA 510(k) clearance in September 2021 and first software-only clearance as an end-to-end digital platform, allowing the company to market a software-as-a-medical service that uses standalone artificial intelligence (AI) and deep learning algorithms to perform remote diagnosis.¹
This digital platform integrates hospital-grade nanosensors, telehealth software and AI-driven decision support that enables providers to remotely triage risk profiles of the cardio, pulmonary and upper vascular systems of patients.
The technology has the ability to save lives because it detects worsening heart failure long before hospitalization would be necessary by delivering an algorithmic score to physicians so they can intervene early. In turn, reduced heart failure hospitalizations reduce health care costs. SimpleSENSE technology is similar to a multi-parameter, multi-vector algorithmic scoring thesis that was validated for FDA clearance in implantable devices, but until now has not been achieved in a noninvasive and cost-effective delivery mechanism.
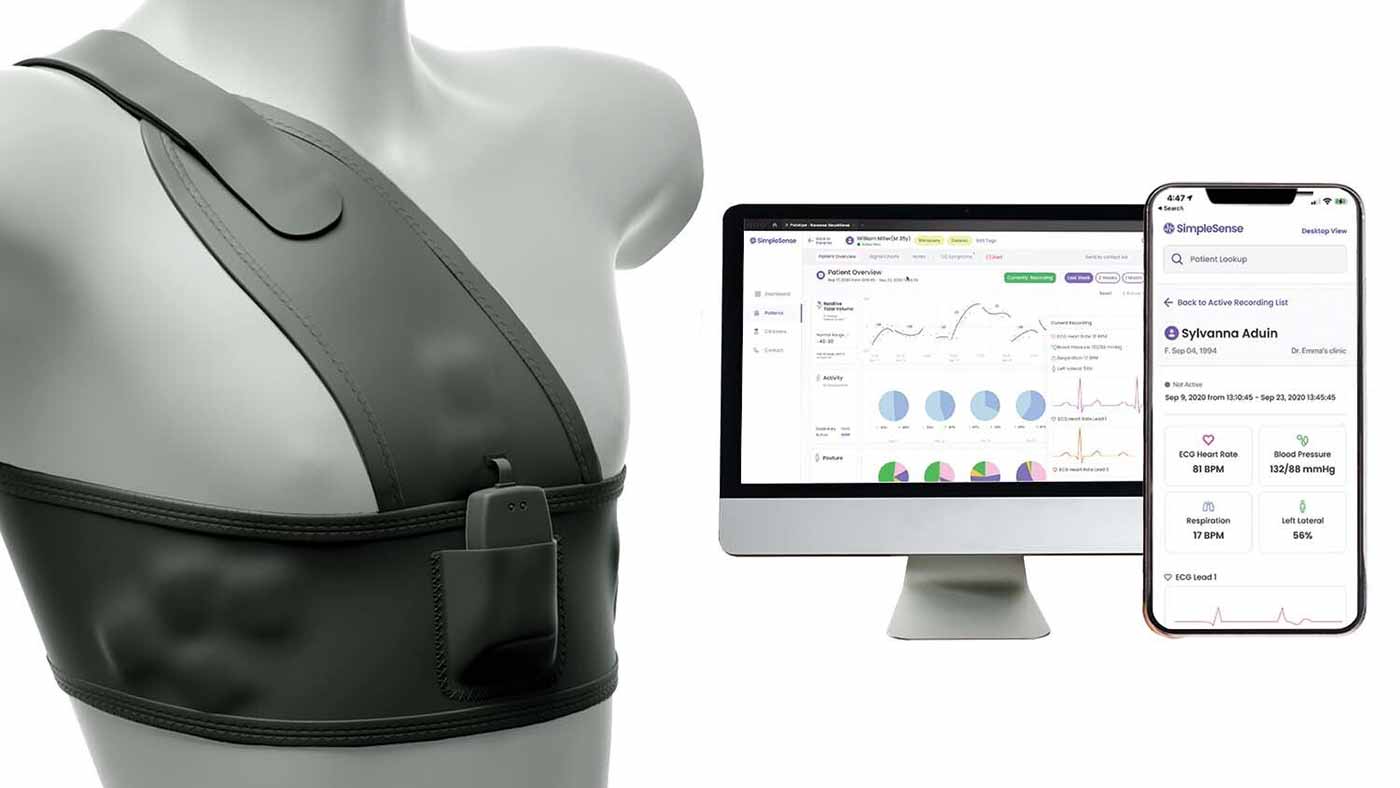
The non-invasive nanowear undergarment monitors multiple patient vitals such as heart rate and sounds, respiration rate, lung volume and physical activity. Nanowear’s patented cloth-based nanosensor technology captures over 120+ million patient data points per day.
“Our goal is to figure out who the high-risk patients are and focus resources on managing them and keeping them out of the hospital,” says Dr. John Boehmer, director of the heart failure program at Penn State Heart and Vascular Institute and the national principal investigator for NanoSENSE. “Putting an implantable device in every patient admitted for heart failure is impractical. What we want is a wearable device that patients can use while they’re at high risk and then move on to more traditional care.”
Study participants wear the SimpleSENSE garment 12 hours per day — divided up into approximately two hours before bed, eight during sleep and two after waking for a total of 90 days. SimpleSENSE nanosensors placed within the fabric use dry-contact electrodes to gather a variety of physiologic data. Researchers will use the data they collect to develop an algorithm that predicts worsening heart failure before symptoms occur, allowing clinicians to manage heart failure more effectively and avoid hospitalizations.
“Measuring how frequently and deeply a patient breathes can be helpful in managing heart failure because many patients with this condition have sleep apnea or another form of sleep-disordered breathing, which would show up in the respiratory data,” Boehmer explains. “Nanowear’s core technology of cloth-based, dry-contact electrodes provides both excellent electrical signals to monitor the heart as well as impedance cardiography and thoracic impedance measurements to monitor both heart and lung function. Combined with heart sounds, activity and posture, a high-fidelity, multisensor recorder can be generated to monitor the heart failure condition.
“Nationally, people discharged from the hospital after an episode of worsening heart failure are readmitted at a rate of 22.5%, or almost 1 in 4,” Boehmer adds. “Despite the fact that we admit heart failure patients to the hospital and treat them, they don’t do well and frequently come back. This contributes to the more than 1 million heart failure-related readmissions annually, costing the U.S. health care system $40 billion every year. Our goal is to figure out who the high-risk patients are and focus resources on managing them and keeping them out of the hospital.”
The SimpleSENSE technology also features an attached electronic unit and a mobile phone for uploading data to the cloud, which may eventually lead to patients accessing and managing their own data.
Boehmer sees the benefits of SimpleSENSE as equal parts patient empowerment and improved heart failure management.
“We want to give patients control of their life, not only so they can know what’s happening when they become symptomatic, but also so they can manage heart failure preemptively.”
Adapted from an article that first appeared in Central Pennsylvania MD News January 2020.

John P. Boehmer, MD
Associate Dean for Faculty Affairs, Penn State College of Medicine
Professor, Department of Medicine
Director, Heart Failure Program, Penn State Heart and Vascular Institute
Phone: 717-531-8407
Email: jboehmer@psu.edu
Fellowship: Cardiovascular medicine, Johns Hopkins University Hospital, Baltimore, Md.
Residency: Internal medicine, University of Massachusetts Medical Center, Worcester, Mass.
Medical School: Penn State College of Medicine, Hershey, Pa.
Connect with John P. Boehmer, MD, on Doximity
Reference
- FDA Clears Nanowear Platform to Implement AI-based Home Diagnostics and Monitoring Network. Diagnostic and Interventional Cardiology. https://www.dicardiology.com/content/fda-clears-nanowear-platform-implement-ai-based-home-diagnostics-and-monitoring-network. Published 2021. Accessed October 6, 2021.
