Technological Advances Lead to Improved Survival with Permanent Left Ventricular Assist Devices
Heart and Vascular Institute Cardiologists Use Latest Tools to Expand Patient Options
Since 1976, when surgeons at Penn State Health Milton S. Hershey Medical Center implanted the first Pierce-Donachy left ventricular assist device (LVAD) in the country, the technology has helped thousands of patients with severe heart failure survive until a donor heart is available for transplant. Today, however, Penn State Heart and Vascular Institute uses LVAD therapy not only as a bridge-to-transplant (BTT), but also as long-term destination therapy (DT). In fact, half of the LVAD placements now performed at Penn State Hershey are for DT usage.
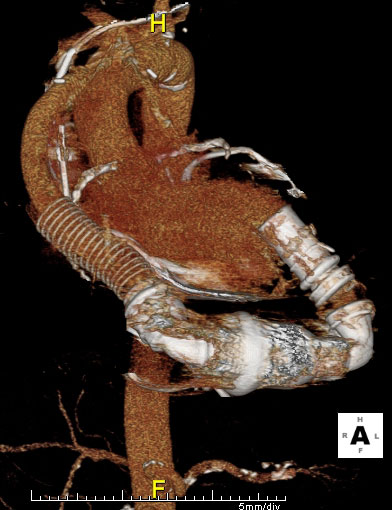
Three-dimensional image of an implanted LVAD.
Although heart transplantation remains the gold standard for advanced heart failure, LVAD can provide a viable option for many. Patients must have three main qualifications to be considered for LVAD therapy: 1) must be in advanced heart failure, but contraindicated for a heart transplant, or too sick to wait for a transplant, 2) must have reached the limits of medical therapy, and 3) must have a life expectancy of more than two years.
The primary limitation of LVAD therapy is the “drive line” which exits the body to connect to an external power source and requires patients to make significant lifestyle adjustments (such as not submerging the device in water). However, fully implantable devices are in the pipeline.
“There has been such rapid progress in this type of technology that even physicians who finished medical school as recently as ten years ago may not realize how much the technology has improved in terms of safety in patients with advanced heart failure,” says Behzad Soleimani, MD, surgical director, transplantation and mechanical circulatory support, Penn State Hershey Heart and Vascular Institute. “This is simply because the progress has been so accelerated with these devices. This technology works. It saves lives.”
This assessment is borne out of results from the HeartMate II trial, in which Penn State Hershey participated, to expand on the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) study, which used the HeartMate I. This trial tested the HeartMate II, the latest version of an LVAD from device manufacturer Thoratec. The device was seen to be superior to medical therapy in terms of one-year survival (52 percent among the trials’ LVAD-treated patients and 25 percent for the patients randomized to optimal medical therapy).1,2 Soleimani summarizes: “It wasn’t a big surprise, but it does show that for DT, as well as BTT, LVAD outperforms the best available medical therapy.”

Behzad Soleimani, MD
Director (interim), Penn State Heart and Vascular Institute
Chief, Division of Cardiac Surgery
Surgical Director, Heart Transplantation and Mechanical Circulatory Support
Professor of Surgery
Phone: 717-531-8330
Email: bsoleimani@pennstatehealth.psu.edu
Fellowship: Cardiopulmonary transplantation surgery, Harefield Hospital, NHS Foundation Trust, Middlesex, England
Residency: Cardiothoracic surgery and general surgery, The Royal College of Surgeons of England, London, England
Medical School: University of Cambridge School of Medicine, Cambridge, England
Connect with Behzad Soleimani, MD, on Doximity
References:
- Park SJ, Milano CA, Tatooles AJ, et al; for the HeartMate II Clinical Investigators. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012;5(2):241-248.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; for the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435-1443.
