Clinical Trials Investigate Novel Treatment Methods in Glioblastoma Multiforme Tumors
Glioblastoma multiforme (GBM) is one of the most medically challenging brain tumors, and can be fatal within one year.¹ Approximately 18,000 patients are diagnosed with GBM in the U.S., annually.² The current standard of treatment—maximal resection, followed by radiation with concurrent and adjuvant temozolomide chemotherapy has shown only modest improvement, with a median survival of 14.6 months, and a two-year survival rate of 26.5 percent.¹ “We need new avenues to improve survival rates,” says Assistant Professor of Neuro-Oncology Dawit Aregawi, MD He explains that Penn State Milton S. Hershey Medical Center is conducting trials in immunologically-mediated therapy among the 90 percent of patients who experience tumor recurrences.³
While past GBM research has focused on chemotherapy, scientists have switched their focus to therapy utilizing the body’s own immune system to create tumor-fighting vaccines. Two upcoming phase 1 trials at Hershey Medical Center, ICT-121 and WT2725, target cancer stem cells traditionally resistant to any form of treatment.
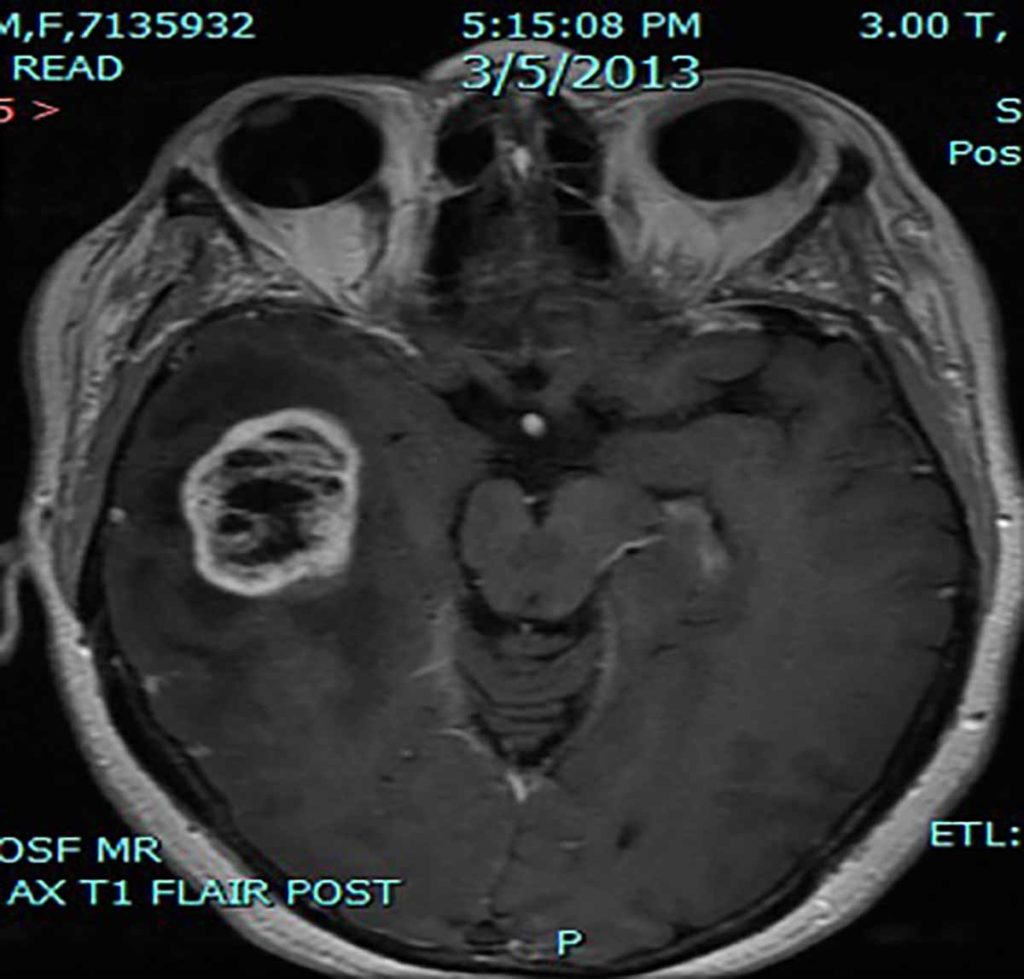
MRI Scan of Glioblastoma multiforme.
In the ICT-121 clinical trial, patients voluntarily undergo apheresis, using their own blood to create autologous dendritic cells, which are mixed with two tumor-stem cell-associated antigens (CD-133+ 405 and 753 peptides) and infused to stimulate their own immune systems and induce cytotoxic T lymphocytes. The primary objective is to assess safety and tolerability of the compound. Using a vaccine, the initial randomized trial showed efficacy in patients with newly diagnosed GBM, and is the first vaccine to demonstrate increased survival. The current study is non-randomized, so that all participants receive active vaccine.
WT2725 is comprised of peptides derived from Wilms Tumor gene product 1 (WT-1) – technically a synthetic protein – with potential immuno-modulating ability, and the WT2725 study tests WT2725 dosing emulsion in patients with advanced malignancies. The primary objectives are to assess safety and tolerability of WT2725 dosing emulsion and determine the maximum tolerated dose based on the evaluation of dose limiting toxicity.
Both are industry-sponsored multi-center phase 1 trials in the recruitment stage, with data collection scheduled to begin over the next year. If the compounds are found to be safe and well-tolerated, phase 2 trials will measure efficacy of tumor response. In addition, Penn State Neuro-Oncology is conducting two trials of the newly-approved non-chemotherapy treatment strategy Optune™, formerly known as NovoTTF™-100A System, a portable medical device that produces alternating electric fields or tumor treating fields (TTFields) and can slow or stop cancer cells from dividing.4
The majority of glioblastomas recur within approximately seven months after standard therapy. While the 2009 Food and Drug Administration’s approval of bevacizumab gave clinicians a useful option for treating recurrences of GBM, the drug was not shown to be effective as an up-front therapy. This research shows promise for up-front treatment, administered with initial standard therapy, to prevent recurrences of these life-threatening tumors.

Dawit G. Aregawi, MD
Assistant Professor of Neurosurgery, Penn State Health Milton S. Hershey Medical Center
Phone: 717-531-3828
Email: daregawi@pennstatehealth.psu.edu
Fellowship: Hematology/Oncology, University of Michigan Medical School, Ann Arbor, Mich.; Neuro-Oncology, University of Virginia Medical Center, Charlottesville, Va.; Neuro-Oncology, National Institutes of Health, Bethesda, Md.
Residency: Internal Medicine, Jewish Hospital of Cincinnati, Cincinnati
Medical School: Jimma Institute of Health Sciences, Ethiopia
Connect with Dawit G. Aregawi, MD, on Doximity
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med 2005;352:987-996.
- neuropathology-web.org/chapter7/chapter7bGliomas.html#gbm. Accessed February 2, 2015.
- medscape.com/viewarticle/540150_2. Accessed February 2, 2015.
- optune.com/therapy.aspx. Accessed February 27, 2015.
